Current Research Projects
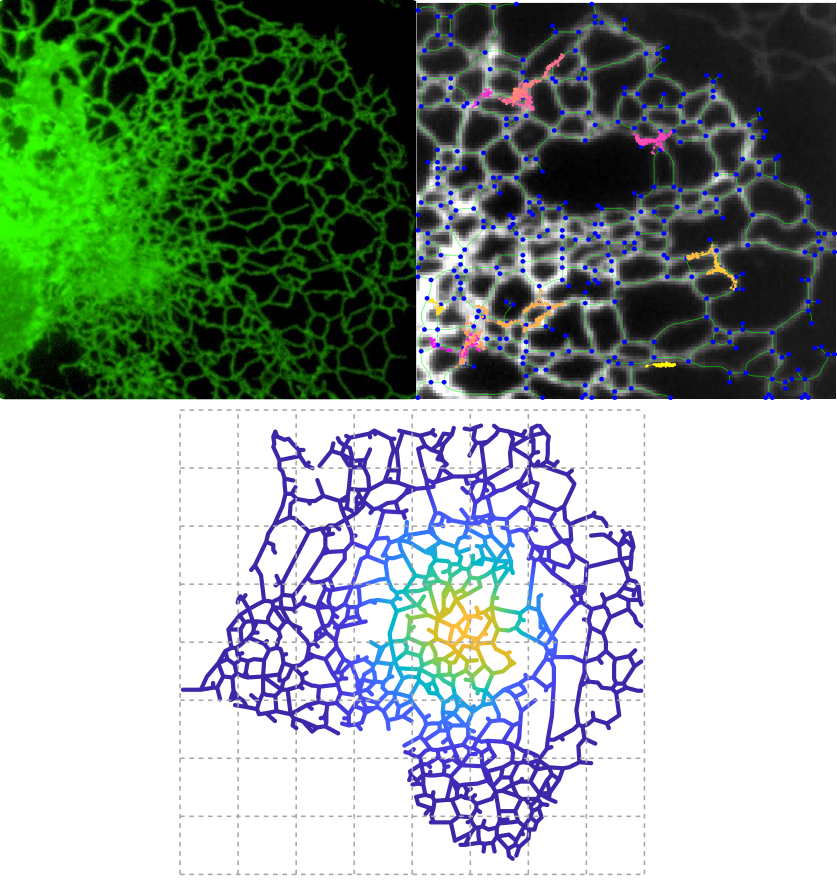
Transport and Morphology in Reticulated Organelles
The endoplasmic reticulum as an intracellular transport network
The endoplasmic reticulum (ER) forms a dynamic interconnected network of perinuclear sheets and peripheral tubules spanning across the cell. The ER serves as a synthesis site for membrane and secretory proteins, plays an important role in protein quality control, and is a key player in intracellular calcium dynamics. We are exploring the structure-function relationship linking ER morphology and its role as a molecular distribution and sorting system. Specific questions include how the connectivity of network structures affects the rates at which proteins can find each other (diffusion-limited kinetics) or target sites in the network (eg: exit sites for leaving the ER); how the structure of the ER is formed dynamically over time; and how higher order structures such as exit sites and ER-mitochondrial contact sites assemble on the ER surface. Furthermore, we investigate the role of ER morphology in calcium signaling events in different cell types. Work on these projects is done in collaboration with several experimental groups: the Avezov Lab, the Westrate Lab, and the Obara Lab.
Papers and preprints:
- ZC Scott, SB Steen, G Huber, LM Westrate, and EF Koslover, “The endoplasmic reticulum as an active liquid network”, PNAS, 121 (42), e2409755121 (2024)
- CC Crapart, ZC Scott, T Konno, A Sharma, P Parutto, DMD Bailey, LM Westrate, E Avezov, and EF Koslover. “Luminal transport through intact endoplasmic reticulum limits the magnitude of localized Ca2+ signals”, Proc Nat Acad Sci, 121(13), e2312172121 (2024)
- T Konno, P Parutto, CC Crapart, V Davi, DM Bailey, MA Awadelkareem, C Hockings, AI Brown, KM Xiang, A Agrawal, JE Chambers, MJ Vander Werp KM Koning, LM Elfari, S Steen, E Metzakopian, LM Westrate, EF Koslover, E Avezov. “Endoplasmic reticulum morphology regulation by RTN4 modulates neuronal regeneration by curbing luminal transport”, Cell Reports, 43(7), (2024)
- ZC Scott, K Koning, M Vanderwerp, L Cohen, LM Westrate, EF Koslover. “ER network heterogeneity guides diffusive transport and kinetics” Biophys J, 122 (2023)
- Z Yang, EF Koslover. “Diffusive exit rates through pores in membrane-enclosed structures”, Phys Biol, 20(2), 026001 (2023)
- Y Sun, Z Yu, CJ Obara, K Mittal, J Lippincott-Schwartz, and EF Koslover. “Unraveling trajectories of diffusive particles on networks” in Phys Rev Res 4.2, 023182 (2022)
- ZC Scott, AI Brown, SS Mogre, LM Westrate, and EF Koslover. “Diffusive search and trajectories on tubular networks: a propagator approach.” in Eur Phys J E, 44(6), 1-20 (2021)
- AI Brown, LM Westrate, and EF Koslover "Impact of global structure on diffusive exploration of organelle networks", in Scientific Reports, 10.1: 4984 (2020)
Spreading and heterogeneity in dynamic networks
Mitochondrial network structure and transport properties
In many cell types, mitochondria fuse into extensive, dynamically rearranging network structures. These network have been proposed to enable homogenization of mitochondrial contents or to provide for selective exclusion of damaged mitochondria; however the overall function of network formation remains unclear. We are exploring how fusion and fission rates couple with mitochondrial transport and mechanics to determine network structure and distribution. In addition, we model the transport of diffusive particles (eg: ions or proteins) through the network, quantifying the extent to which network dynamics and density govern the rate of material mixing versus maintenance of heterogeneity between mitochondria.
Papers and preprints:
- KB Holt, C Zurita, L Teryoshin, SC Lewis, and EF Koslover, “Diffusive Spreading Across Dynamic Mitochondrial Network Architectures", arXiv preprint arXiv:2506.05643., 2025
- KB Holt, J Winter, S Manley, EF Koslover, “Spatiotemporal Modeling of Mitochondrial Network Architecture.”, PRX Life, 2(4), 043002 (2024)
- MP Viana, AI Brown, IA Mueller, C Goul, EF Koslover, and SM Rafelski "Mitochondrial Fission and Fusion Dynamics Generate Efficient, Robust, and Evenly Distributed Network Topologies in Budding Yeast Cells", in Cell Systems, 10.3: 287-297 (2020)
- AI Brown, LM Westrate, and EF Koslover "Impact of global structure on diffusive exploration of organelle networks", in Scientific Reports, 10.1: 4984 (2020)
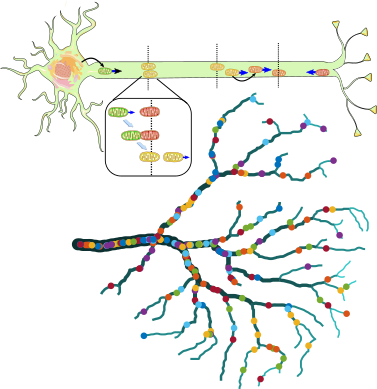
Dynamics and Distribution of Neuronal Organelles
Contact dynamics and signal propagation among motile organelles
Recent work has highlighted a plethora of inter-organelle contacts in mammalian cells and has begun to unravel the many biological functions served by such interactions. In the case of globular organelles such as lysosomes, endosomes, phagosomes, peroxisomes, and neuronal mitochondria, intracellular transport is required for the dynamic formation of such contacts. Our group is exploring how transport systems modulate inter-organelle contact events in several different contexts, with a focus on long cellular projections such as neuronal axons. One thread of interest is understanding the maturation of axonal autophagosomes as they fuse with passing lysosomes during their retrograde journey to the cell body for recycling. Another thread focuses on the distribution and maintenance of mitochondria in neurites. In particular, we expore how even distribution of mitochondria throughout the dendritic arbor is supported by specific morphological scaling laws and how distally stationed mitochondria can be maintained in the face of protein turnover by transient interactions between a stationary and a motile pool. Furthermore, we are investigating the structure of the mitochondrial population when individual units can fuse into intermediate-size clusters, and the role of fusion, fission, and transport dynamics in governing the propagation of signals through the 'social network' of mitochondria. Quantitative analysis of mitochondrial distribution and material exchange in the dendritic arbors of Drosophila sensory neurons is compared against model predictions, with the aid of collaborators in the Barnhart group.
Papers and preprints:
- M Hidalgo-Soria and EF Koslover, “Clustering and spatial distribution of mitochondria in dendritic trees”, Phys Rev Res, 6.4, 043312 (2024)
- EJ Donovan, A Agrawal, N Liberman, JI Kalai, AJ Adler, AM Lamper, HQ Wang, NJ Chua, EF Koslover, and EL Barnhart. “Dendrite architecture determines mitochondrial distribution patterns in vivo”, Cell Reports, 43(5), (2024)
- SE Cason, SS Mogre, EF Koslover, ELF Holzbaur. “Neuronal Autophagy by the Numbers”, Autophagy Reports, 2(1), (2023)
- SE Cason, SS Mogre, ELF Holzbaur, EF Koslover. “Spatiotemporal analysis of axonal autophagosome–lysosome dynamics reveals limited fusion events and slow maturation”, Molecular Biology of the Cell 33 (13), ar123 (2022)
- A Agrawal and EF Koslover. “Optimizing mitochondrial maintenance in extended neuronal projections.” in PLoS Comp Biol 17.6: e1009073 (2021)
- A Agrawal, G Pekkurnaz, and EF Koslover “ Spatial control of neuronal metabolism through glucose-mediated mitochondrial transport regulation”, in ELife, 7: e40986 (2018)
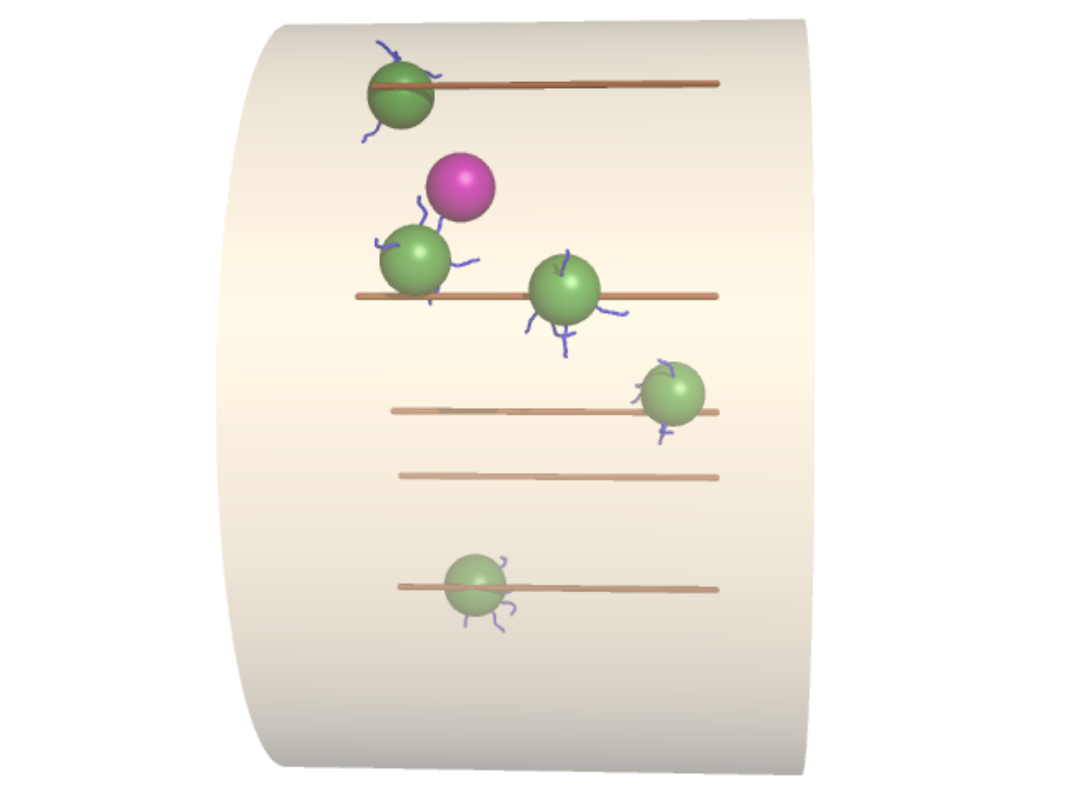
Physics of Intracellular Active Transport
Spatial organization and mechanics of motor-driven transport in eukaryotes
Eukaryotic cells must transport components ranging from small molecules to micron-sized vesicles through a complex and heterogeneous physical environment. To aid in this task they employ active transport -- the directed motion of motors along cytoskeletal highways to carry vesicular cargo. While some cargoes are linked directly to molecular motors, others "hitchhike" by indirect attachment to carrier vesicles. Some maintain a high affinity for microtubules, lying in wait for attachment to a passing motor, while others undergo diffusion, driven by thermal or actively generated fluctuations that aid in dispersion in between active motion events. We are investigating the mechanics and dynamics of these transport processes, using a combination of simulations and analytical theory for multi-modal transport phenomena, working in collaboration with experimental researchers who track organelle motion in live cells. Topics of interest include: the efficiency of dispersion by multi-modal transport mechanisms at whole-cell scales and the effect of microtubule positioning on cargo transport
Papers and preprints:
- SS Mogre, JM Christensen, SL Reck-Peterson, and EF Koslover. ``Optimizing microtubule arrangements for rapid cargo capture." in Biophys J, 120.22, 4918-4931 (2021)
- SS Mogre, AI Brown, and EF Koslover “Getting around the cell: physical transport in the intracellular world.”, in Phys Biol 17.6, 061003 (2020)
- SS Mogre, JR Christensen, CS Niman, SL Reck-Peterson, and EF Koslover "Hitching a Ride: Mechanics of Transport Initiation through Linker-Mediated Hitchhiking", in Biophys. J., 118.6: 1357-1369 (2020)
- SS Mogre and EF Koslover “Multimodal transport and dispersion of organelles in narrow tubular cells ”, in Phys. Rev. E, 97.4: 042402 (2018)

Exploratory dynamics of biomolecular systems
Functional consequences of breaking equilibrium in reaction circuits
Dynamic processes at the molecular and cellular scale can often be coarse-grained to systems of discrete Markovian states, undergoing `exploratory dynamics' that involve sampling many paths before reaching a target. We investigate how milestone states can be defined in complex spatiotemporal systems to allow this coarse-grained analysis. In a variety of biological contexts, this approach enables us to explore how breaking detailed balance leads to the formation of driven cycles and modulates the behavior of the system. Examples include cyclical glycosylation for protein quality control, kinetic proofreading during translation, localization of mitochondrial mRNAs, and cargo transport on randomly distributed microtubule arrays. All of these systems exhibit `exploratory dynamics', sampling over many paths to reach a given target. For many of them, deviation from equilibrium enhances the accuracy and signal-sensitivity of the system in question.
Papers and preprints:
- EF Koslover, MM Lin, R Phillips, “Many Will Enter, Few Will Win: Cost and Sensitivity of Exploratory Dynamics." arXiv preprint arXiv:2506.00775.
- XG Arceo, EF Koslover, BM Zid, AI Brown. “Mitochondrial mRNA localization is governed by translation kinetics and spatial transport”, PLoS Comp Biol 18 (8), e1010413 (2022)
- ZC Scott, AI Brown, SS Mogre, LM Westrate, and EF Koslover. “Diffusive search and trajectories on tubular networks: a propagator approach.” in Eur Phys J E, 44(6), 1-20 (2021)
- AI Brown, and EF Koslover “Design principles for the glycoprotein quality control pathway”, in PLoS Comp Biol 17.2, e1008654 (2021)
- Z Chen, R Gabizon, AI Brown, A Lee, A Song, C Diaz-Celis, CD Kaplan, EF Koslover, T Yao, C Bustamante “High-resolution and high-accuracy topographic and transcriptional maps of the nucleosome barrier”, in ELife, 8: e48281 (2019)